Manganese (Mn) forms around 0.1 % of the earth’s crust, making it a common metal. Mn is found in soil at an average concentration of approximately 440 ppm, though this can range from 7 to 9000 ppm depending on the soil type being analyzed. The Thermo ScientificTM NitonTM series of handheld XRF analyzers can easily accommodate these concentrations.
Mn ores are generally comprised of dark brown to black oxides, most notably pyrolusite (MnO2) and psilomelane (BaMn9O18 2H2O). Manganese silicate (braunite, MnSiO3) and manganese carbonate (rhodochrosite, MnCO3) sometimes occur concomitantly. Mn minerals typically exhibit a close spatial relation to iron (Fe) ores.
Mn is mined in several different countries, including India, Australia, China, Brazil, Gabon, South Africa, Ukraine, Ghana, and Kazakhstan.
Handheld XRF Analyzers in Mining
X-ray fluorescence (XRF) is an analytical technique that delivers rapid, accurate elemental analysis with minimal—or even no—sample preparation.
XRF can be applied in different stages of mining activity, including grass-roots exploration, exploitation, ore grade control, and environmental investigations.
The Niton range of handheld XRF analyzers can detect elements ranging from magnesium (Mg) to uranium (U). These analyzers provide accurate results over numerous samples while offering low limits of detection, leading to transformative improvements in data acquisition time.
Application
The chemical properties of Mn are very similar to those of Fe. Mn, therefore, accompanies Fe ores, and small amounts of Mn are frequently found substituting for Fe in iron-based minerals.
Different types of Mn deposits can be found globally. These include Precambrian Mn formations (over 541 million years old), shallow marine deposits, black shale-hosted MnCO3 deposits, high-grade ores developed from low-grade ore (supergene deposits), and Mn crusts and nodules.
Around several billion tons of unmined Mn nodules are present on ocean floors. Approximately 85–90 % of global Mn consumption occurs in the steel industry, where Mn is used to form ferromanganese alloys.
Mn is also routinely used in the chemical industry, with common applications, including potassium permanganate for water treatment and purification and MnO2 in dry cell batteries.
Method
The case study presented here was performed using a Thermo ScientificTM NitonTM XL3t GOLDD Analyzer with a Mining Mode calibration. Analysis was completed for seconds (30 seconds for the main filter and 20 seconds for each other filter).
A total of 33 Fe-Mn ore samples acquired from a Fe-Mn mine site were analyzed. Lab assays for 14 of these samples are included for comparison purposes.
Figure 1 shows the sample concentrations of Fe, Mn, and Si (as a light element), along with the correlating lab assay data.
One of the samples was also analyzed eight times under identical conditions to assess the assay’s repeatability (precision). Figure 2 shows these repeatability results.
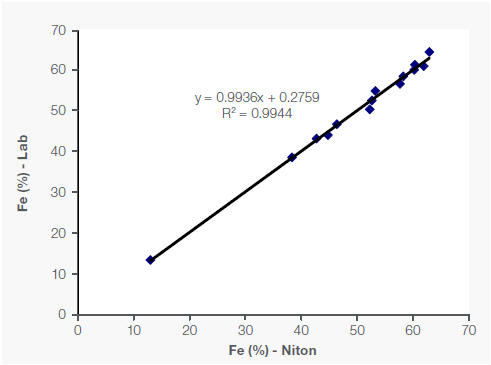
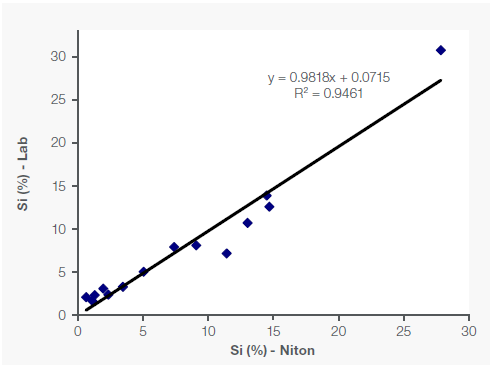
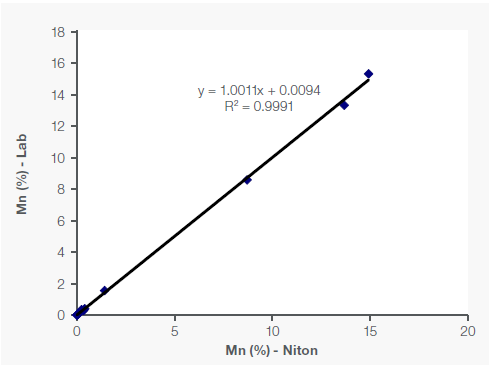
Figure 1. Correlation of Fe, Mn and Si data between portable XRF and lab in Fe-Mn ore samples. Image Credit: Thermo Fisher Scientific – Handheld Elemental & Radiation Detection
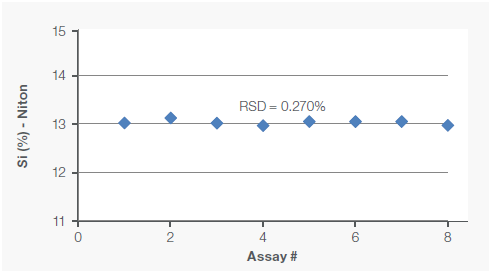
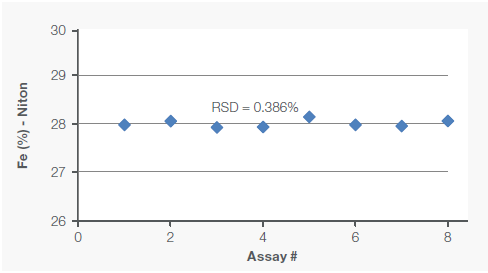
Figure 2. Repeatability (precision) of Fe and Si assays was tested by analyzing one sample eight times. Image Credit: Thermo Fisher Scientific – Handheld Elemental & Radiation Detection
Results
The R² value (coefficient of determination) measures how closely the data sets correlate with one another, with a perfect correlation equaling an R² of 1. In the example presented here, the correlation for Fe and Mn was robust, with an R² of over 99%, while the R² was 95% for Si (Figure 1).
Assay data also showed high precision, as demonstrated by the low relative standard deviation of 0.386 % for Fe and 0.270 % for Si.
Conclusion
Handheld XRF analyzers offer real-time and non-destructive elemental analyses for around 30 elements ranging from Mg to U. These instruments are ideally suited to supporting geologists in the timely process and planning decisions required in the field.
These analyzers reliably evaluate many sample types, whether encountered in the exploration stage (low grade) or the ore grading stage (high grade). High threshold concentrations of both Fe and Mn in natural samples indicate that this technique can reliably provide accurate, precise data in Fe-Mn exploration and mining.
Acknowledgments
Produced from materials originally authored by Thermo Scientific.

This information has been sourced, reviewed, and adapted from materials provided by Thermo Fisher Scientific – Handheld Elemental & Radiation Detection.
For more information on this source, please visit Thermo Fisher Scientific – Handheld Elemental & Radiation Detection.